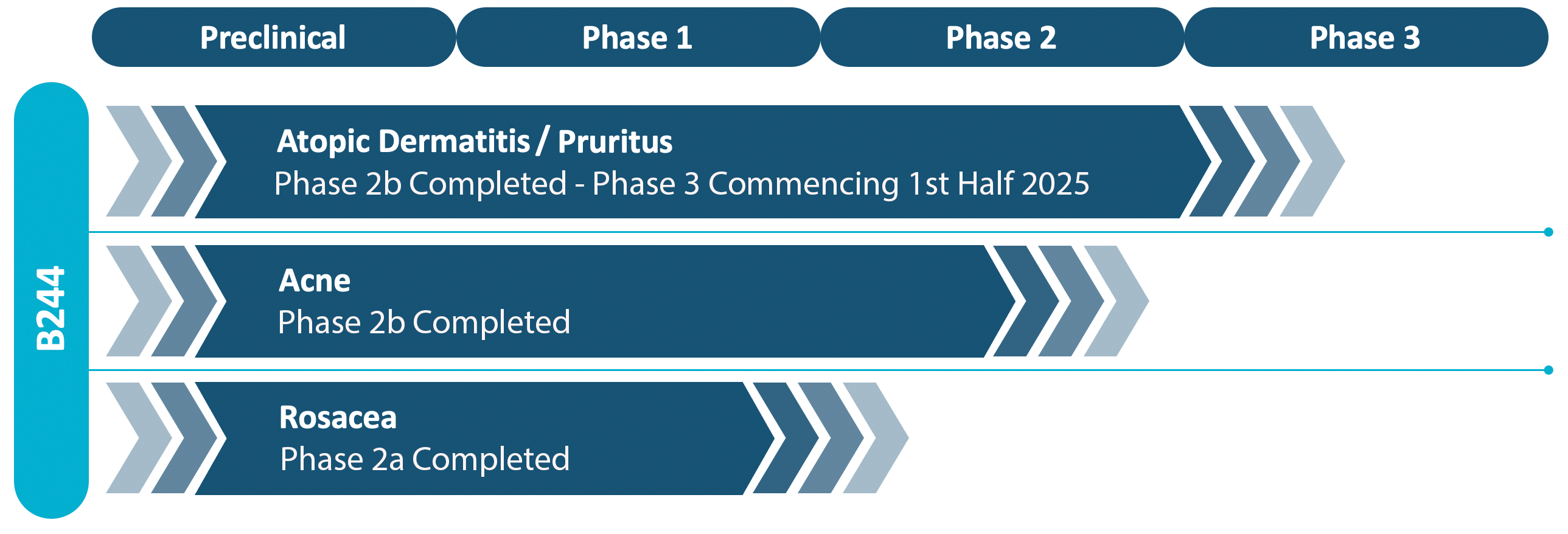
We have completed six Phase 2 clinical programs and multiple pre-clinical programs. Over 1,700 patients have been enrolled in our clinical trials since 2016. Regardless of dose, mode of delivery, indication targeted, or age of patients, our bacterial drug candidate B244 has been very well tolerated with an adverse event profile that is equal to or less than the vehicle, which is essentially saltwater (PBS buffer).
The company has selected its lead candidate based upon pre-clinical and clinical results from those trials. The company has completed it’s Phase 2b trial with 547 patients in a double blind placebo controlled trial for the treatment of adults with pruritus associated with atopic dermatitis in support of its lead program. Other programs, moderate to severe acne, will be pursued at a later date.
The following diagram shows the current stage of development of our product pipeline: